The adaptive immune system mounts pathogen-specific humoral and cellular responses to combat infections. Upon identification of a new virus, B- and T-cells will elicit specific responses to the infection.
A new PNAS journal study reports that the reduced efficiency of the immune response against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could be due to the reduced diversity of both B- and T-cells. This reduction in T-cell diversity was observed only in subjects over 50 years of age who are at an increased risk of coronavirus disease 2019 (COVID-19) morbidity and mortality.
Such a discrepancy in how older persons mount T cell responses to a new virus may be considered a risk factor for the elderly, particularly vis-à-vis new virus variants against which T cell immunity may be particularly important.”
Study: Global Patterns of Antigen Receptor Repertoire Disruption Across Adaptive Immune Compartments In COVID-19. Image Credit: bankbee / Shutterstock.com
Introduction
The occurrence of severe COVID-19 following SARS-CoV-2 infection has been associated with several factors, such as advancing age. Age-associated immune dysregulation or immunodeficiency is assumed to be responsible for this.
The adaptive immune system provides specific protection to the host against SARS-CoV-2. Simultaneously, the immune system may generate autoimmune antibodies, as well as reactivating cross-reactive and non-protective memory B-cells.
With age, both CD4 and CD8 T-cell receptors (TCRs) exhibit a reduced diversity of sequences. This may be why older people are often susceptible to several newer viral diseases, including those caused by SARS-CoV, SARS-CoV-2, and the West Nile virus.
To explore this association, the investigators used immunoPETE, which is a novel genomic DNA (gDNA) technique where all major T- and B-cell receptors are characterized for their range of antigen recognition. This includes B-cells (immunoglobulin heavy chain [IGH] sequences); αβ T-cells (TCRβ chain and TRB sequences) composed of CD4+ and CD8+ cells; and γδ T-cells (TCRδ chain and TRD sequences), which comprises Vδ1+ and Vδ2+ T-cells.
Study findings
In a set of almost 100 subjects, including seropositive and seronegative individuals, as well as hospitalized COVID-19 patients, the IGH repertoire expanded, with SARS-CoV-2-related clusters of IGH sequences increasing in relation to seroconversion. Similar expansions were observed with the TRB and TRD repertoires; however, significant disruption in diversity in those above the age of 50 years was observed.
As a result of the IGH enrichment, clonal expansion appeared to have occurred within two weeks of symptom onset. By day 50, this effect was significant only in those younger than 50 years of age. There was no observable association with any age, disease severity, or clinical features.
Clusters of structurally-related IGH sequences that had common specificities were stronger in younger patients across the spectrum of disease severity. This indicates a loss of diversity of IGH sequence clusters in SARS-CoV-2-exposed subjects.
This was due to the emergence of SARS-CoV-2-reactive B-cell clones, as dozens of exact matches between the IGH sequences and those available on the CoV-AbDab database of SARS-CoV-2 reactive immunoglobulins (Igs) were identified. These sequences were expanded in people with active COVID-19 as compared to seronegative people.
Strong correlations between IGH focusing and plasmablast frequency, as well as IgM antibodies to SARS-CoV-2 proteins, were also observed. However, the CDR3-CARGFDYW sequence, which was most often shared between study subjects and the CoV-AbDab database, occurred only in SARS-CoV-2-exposed individuals and did not elicit non-neutralizing antibodies to the spike protein.
As for TRB, the scientists did not find any evidence that T-cells expressing specific Vβ genes were being targeted by viral superantigens for polyclonal activation that would subsequently be responsible for both COVID-19 and multisystem inflammatory syndrome in children (MIS-C).
Several Vβ cells were linked to significant changes, especially TRBV6-4, which exhibited a significant reduction in its frequency among subjects with active COVID-19. This was likely because TRBV6-4 is most richly expressed by CD8 mucosal-associated invariant T-cells, which also exhibited a sharp decline in their frequency during COVID-19.
TCRVβ was found to be related to patient age in COVID-19. The most significant narrowing of CD4 TRB repertoires was observed among those over the age of 50 who were exposed to SARS-CoV-2 during the first two weeks following symptom onset.
The same trend was observed among CD8 TRB repertoires in the same patient population. However, this was a function of both age and disease, rather than due to either of these factors alone, with both repertoires showing strongly correlated focusing. In all analyses, a renormalization of TRB repertoires appeared to occur over time in older patients.
TCR clusters are formed by TCRs that respond specifically to SARS-CoV-2 antigens. Thus, most clusters would be found in samples obtained from SARS-CoV-2-exposed individuals. However, in the current study, although TRB repertoires were focused in an exaggerated manner in older people, unique TRB repertoires formed clusters in equal proportions in both age groups.
The inference is that older people experience greater disruption of their global repertoires as compared to younger patients, though both eventually reach the same endpoint. This could be due to the increased expansion of non-virus-specific TCRs with age. Similar changes were reported with the αβ T-cell response.
The researchers hypothesize that Vδ1+ TCRs react to multiple molecular signals of immune dysregulation induced by SARS-CoV-2, rather than to specific viral antigens.
Vδ2+ T-cells were prominent in peripheral blood; however, these cells did not experience narrow clonal expansion following SARS-CoV-2 exposure. This observation supports their role in innate-like immunity, which contrasts with the activity of Vδ1+ T-cells that have a more dominant role in adaptive immune responses.
Overall, COVID-19 appears to be related to a significant narrowing of the IGH repertoire, whereas global disruptions of αβ and γδ T-cell repertoires were dominant in older subjects of either sex.
Implications
The current study measured adaptive immune responses to a known challenge occurring at a known time point against a background of immune dysregulation. The results show that SARS-CoV-2 elicited antigen-specific responses, irrespective of age and clinical severity of disease.
B-cell repertoires appeared to respond in a comparable fashion in all groups. However, T-cell repertoires exhibited an unexpected disruption at and after the age of 50, which may support earlier evidence of age-related unpredictable dysregulation of the adaptive immune response in COVID-19.
Such impacts may limit the capacity to recognize diverse challenges (e.g., coinfections and emerging new variants) and may afford undue prominence to expanding nonneutralizing and/or self-reactive clones. Thus, they should be considered an additional age-related disease risk.”
These findings have also revealed adaptive Vδ1 responses in the context of live SARS-CoV-2 challenge; however, this response occurred without any sequence sharing associated with the virus. Thus, Vδ1+ cell expansions may be the result of reactivity to SARS-CoV-2-induced changes in the expression of endogenous antigens, rather than that of the viral antigens.
The mechanisms responsible for the disruption of T-cell repertoires with age may include the greater proportion of senescence-associated CD57+ CD28−p16+ T-cells that are slow to undergo clonal expansion. Additionally, the expansion of naïve antigen-specific T-cells to counter this new pathogen from an unpredictable pool of naïve T-cells in older people may also be a contributing factor.
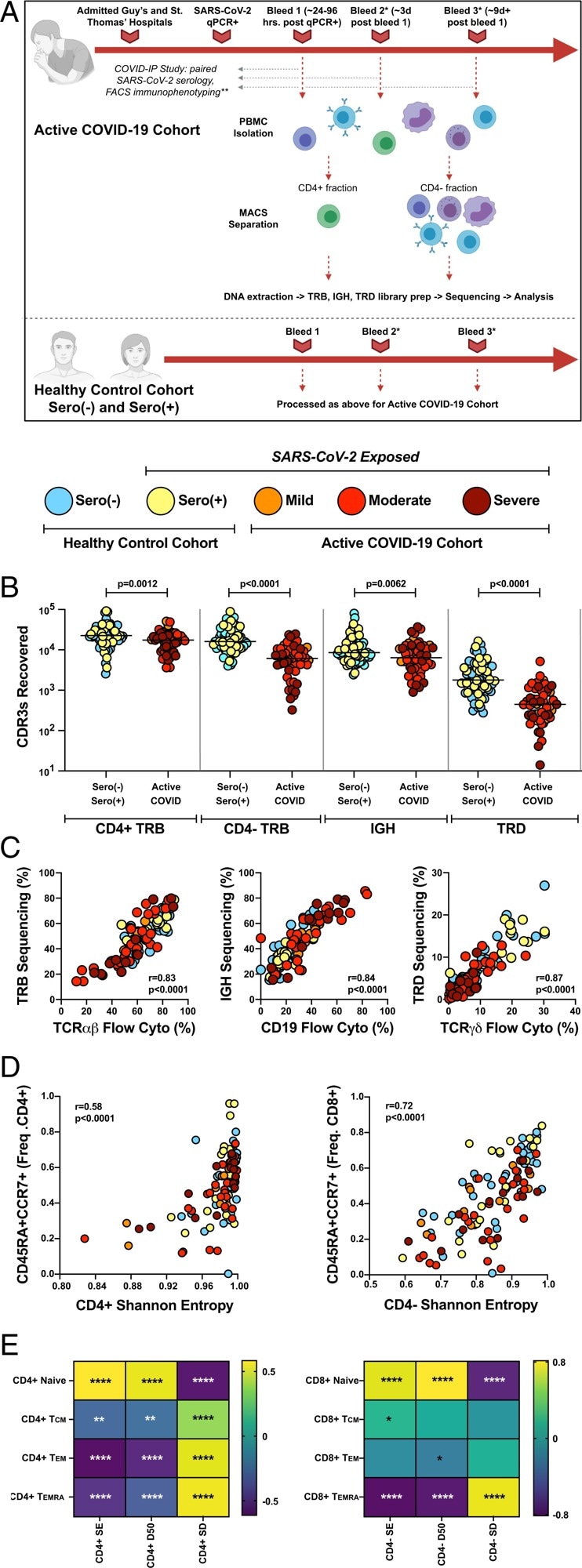
Application of immunoPETE to the COVID-IP Study cohort enables efficient, high fidelity, and quantitative recovery of TRB, IGH, and TRD CDR3s from peripheral blood mononuclear cells (PBMCs). (A) Summary of workflow for samples recruited into the present study. MACS: magnetic-activated cell sorting. *Not all donors had longitudinal blood sampling. **Not all healthy control samples were run through the full COVID-IP Study pipeline. All samples had SARS-CoV-2 serology data. (B) Recovery of CDR3s from sero(–) (n = 47), sero(+) (n = 26), mild (n = 10), moderate (n = 26), and severe (n = 16 CD4+; n = 15 CD4− fraction) samples by immunoPETE. gDNA (2 × 250-ng replicates) was used for CD4+ library preparation (prep) and 1,000 ng of gDNA (4 × 250-ng replicates) was used for CD4− library preparation. Bar indicates the median value. Mann–Whitney U test results are shown. (C) Correlation between immunoPETE and flow cytometry (cyto). Percentage of TRB, IGH, and TRD CDR3s recovered by immunoPETE sequencing in the CD4− fraction (y-axis) versus percentage of CD3+ (n = 112), CD19+ (n = 112), and TCRγδ+ (n = 112) assayed by flow cytometry (x-axis) of the same sample. Spearman’s correlation was used for analysis. (D) Correlation of overall CD4+ TRB (Left) and CD4− TRB (Right) diversity with frequency (freq) of naïve (CD45RA+CCR7+) CD4+ (n = 107) or CD8+ T cells (n = 106), respectively, as previously reported in the COVID-IP Study. Spearman’s correlation was used for analysis.
Other possible explanations include the recruitment of pre-existing T-cells primed by cross-reactive antigens from seasonal coronaviruses, which were more likely to have already infected older people over the years. This hypothesis is supported by previous studies showing an increase in cross-reactive memory B-cells during the early humoral response. In contrast, the later disease phase was characterized by neutralizing B-cells that produced anti-receptor-binding domain (RBD) antibodies.
Advancing age beyond 50 years could be a significant risk factor for impaired T-cell responses to new pathogens, as demonstrated by the increased risk of these individuals to COVID-19-related severe and fatal illness. In the current study, older people showed similarly increased risk when exposed to the West Nile virus or SARS-CoV for the first time.
Cancer patients share this trait with older people, as demonstrated by their reduced immune response when vaccinated against new pathogens, including SARS-CoV-2. This is in contrast to the persistent memory T-cell responses to previously encountered pathogens in the same subjects.
Thus, the higher T-cell immune response could lead to reduced TCR diversity, with accompanying vulnerability to neoantigens and emerging variants. Undue T-cell repertoire narrowing would prevent appropriate responses to SARS-CoV-2 antibody escape variants or other coinfecting viruses.
Another undesirable consequence could be an unduly large expansion of non-neutralizing or immunopathogenic immune responses. Thirdly, in the current set of COVID-19 patients, TCR repertoire disruptions accompanied significant age-related declines in Vδ2 sequences, which have an innate-like role in maintaining the immune response to phosphoantigens that serve as markers of infection by many viruses and bacteria.
- Joseph, M., Wu, Y., Dannebaum, R., et al. (2022). Global Patterns of Antigen Receptor Repertoire Disruption Across Adaptive Immune Compartments In COVID-19. PNAS. doi:10.1073/pnas.2201541119.
"severe" - Google News
August 15, 2022 at 08:23AM
https://ift.tt/ZjCvOxA
Why is COVID-19 more severe in people older than 50? - News-Medical.Net
"severe" - Google News
https://ift.tt/gmpxlu0
Shoes Man Tutorial
Pos News Update
Meme Update
Korean Entertainment News
Japan News Update
Bagikan Berita Ini















0 Response to "Why is COVID-19 more severe in people older than 50? - News-Medical.Net"
Post a Comment